Interchalcogen Contents Known binary interchalcogens Bonding in the binary interchalcogens Summary of known binary interchalcogens See also Notes References Navigation menu
InterchalcogensChalcogens
chalcogenselectropositiveelectronegativehalogenfluorineoxygenpoloniumgaseousnonmetalpost-transition metalcovalentionicmetallicsemimetalliccovalent bondingmoleculesionic bondingionic bondingmetallic bondingpolarpolymersfluorite
The chalcogens react with each other to form interchalcogen compounds.[1]
Although no chalcogen is extremely electropositive,[note 1] nor quite as electronegative as the halogen fluorine (the most electronegative element), there is a large difference in electronegativity between the top (oxygen = 3.44 — the second most electronegative element after fluorine) and bottom (polonium = 2.0) of the group. Combined with the fact that there is a significant trend towards increasing metallic behaviour while descending the group (oxygen is a gaseous nonmetal, while polonium is a silvery post-transition metal[note 2]), this causes the interchalcogens to display many different kinds of bonding: covalent, ionic, metallic, and semimetallic.[note 3][1]
Contents
1 Known binary interchalcogens
2 Bonding in the binary interchalcogens
3 Summary of known binary interchalcogens
3.1 Sulfur chalcogenides
3.2 Selenium chalcogenides
3.3 Tellurium chalcogenides
3.4 Polonium chalcogenides
4 See also
5 Notes
6 References
Known binary interchalcogens
| O | S | Se | Te | Po | |
|---|---|---|---|---|---|
| O | O2, O3, O4, O8 | ||||
| S | S2O, SO, S2O2, SO2, SO3 | S2, S3, S6, S7, S8, S∞ | |||
| Se | SeO2, SeO3 | SexSy | Se6, Se7, Se8, Se∞ | ||
| Te | TeO, TeO2, TeO3, Te2O5 | TexSy | TexSey | Te∞ | |
| Po | PoO, PoO2, PoO3 | PoS | PoxSey | PoxTey | Po∞ |
Bonding in the binary interchalcogens
Going down the above table, there is a transition from covalent bonding (with discrete molecules) to ionic bonding; going across the table, there is a transition from ionic bonding to metallic bonding. (Covalent bonding occurs when both elements have similar high electronegativities; ionic bonding occurs when the two elements have very different electronegativities, one low and the other high; metallic bonding occurs when both elements have similar low electronegativities.) For example, in the leftmost column of the table (with bonds to oxygen), O2 and O3 are purely covalent, SO2 and SO3 are polar molecules, SeO2 forms chained polymers (stretching in one dimension), TeO2 forms layered polymers (stretching in two dimensions), and PoO2 is ionic with the fluorite structure (spatial polymers, stretching in three dimensions); in the bottom row of the table (with bonds to polonium), PoO2 and PoS are ionic, PoxSey and PoxTey are semimetallic, and Po∞ is metallic.[1]
Summary of known binary interchalcogens
Sulfur chalcogenides

Molecular structure of sulfur monoxide.
Lower sulfur oxides, SxOy where the ratio X:Y is greater than 1:2
Disulfur monoxide, S2O
Disulfur dioxide, S2O2
Sulfur monoxide, SO
Sulfur dioxide, SO2
Sulfur trioxide, SO3
Higher sulfur oxides, SOx where x>3
Selenium chalcogenides

Molecular structure of selenium trioxide.
Selenium dioxide, SeO2
Selenium trioxide, SeO3- Many "alloys" of selenium and sulfur in different concentrations with semimetallic bonding, SexSy
- "Selenium monosulfide", SeS
- "Selenium disulfide", SeS2, actually a 2:1 mixture of cyclo-Se3S5 and cyclo-Se2S6
- "Selenium trisulfide", SeS3, actually occurring as the cyclic dimer Se2S6
Tellurium chalcogenides
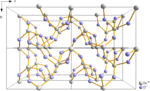
Crystal structure of tellurium dioxide.
Tellurium monoxide, TeO (unstable transient species)
Tellurium dioxide, TeO2
Tellurium trioxide, TeO3
Ditellurium pentoxide, Te2O5[2]- Many "alloys" of tellurium and sulfur in different concentrations with semimetallic bonding, TexSy
- Many "alloys" of tellurium and selenium in different concentrations with semimetallic bonding, TexSey
Polonium chalcogenides
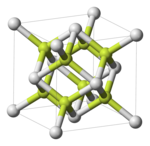
Unit cell of polonium dioxide (cubic modification). Po: white; O: yellow.
Polonium monoxide, PoO
Polonium dioxide, PoO2
Polonium trioxide, PoO3
Polonium monosulfide, PoS- Many "alloys" of polonium and selenium in different concentrations with semimetallic bonding, PoxSey
- Many "alloys" of polonium and tellurium in different concentrations with semimetallic bonding, PoxTey
See also
- Interhalogen
- Hydrogen chalcogenide
Notes
^ This article uses Pauling electronegativity throughout.
^ The classification of polonium as a post-transition metal or a metalloid is disputed.
^ The heavier halogens are sufficiently electronegative to prevent ionic or metallic bonding in the interhalogens, and the lighter pnictogens are not sufficiently electronegative to allow ionic or metallic bonding in the interpnictogens.
References
^ abc Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils, ed., Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, pp. 585–586, ISBN 0-12-352651-5.mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ http://scripts.iucr.org/cgi-bin/paper?S0567740873003092
